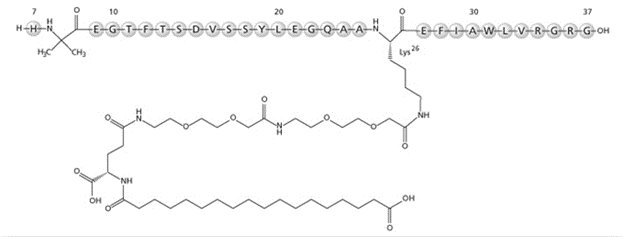
Semaglutide
What is Ozempic (Semaglutide)?
Approved To Treat
Related Clinical Trials
Summary: The study plans to learn more about what happens to the body after bariatric surgery in people 12 to 24 years old. The study aims to understand why people respond differently to bariatric surgery and how to define success beyond weight loss alone. The study also plans to learn more about whether a medication (semaglutide) can help people 12 to 24 years old who, between 1 and 2 years after bariatri...
Summary: Semaglutide (a GLP-1RA) is approved for type 2 diabetes mellitus T2DM and obesity, but its effect on obstructive sleep apnea OSA remains unclear. To evaluate changes in Apnea-Hypopnea Index (AHI) after 1-week and 4-week semaglutide treatment in T2DM/obesity patients with OSA, we conducted a single-center real-world study (RWS) of 15 patients. Outcomes assessed included AHI, weight, BMI, blood pres...
Summary: The primary objective of Study GZQG is to compare the effect of retatrutide and placebo on total clamp disposition index (cDI) after 28 weeks of treatment.
Related Latest Advances
Brand Information
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether OZEMPIC causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- OZEMPIC is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) in adults with type 2 diabetes mellitus and established cardiovascular disease.
- OZEMPIC has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis
- OZEMPIC is not indicated for use in patients with type 1 diabetes mellitus.
- A personal or family history of MTC or in patients with MEN 2
- A serious hypersensitivity reaction to semaglutide or to any of the excipients in OZEMPIC. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with OZEMPIC
- In addition to the reactions in Table 1, the following gastrointestinal adverse reactions with a frequency of <5% were associated with OZEMPIC (frequencies listed, respectively, as: placebo; 0.5 mg; 1 mg): dyspepsia (1.9%, 3.5%, 2.7%), eructation (0%, 2.7%, 1.1%), flatulence (0.8%, 0.4%, 1.5%), gastroesophageal reflux disease (0%, 1.9%, 1.5%), and gastritis (0.8%, 0.8%, 0.4%).
- This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 03/2022




